1st OPEN CALL – (25 July – 30 September 2020) - Remote access to transmission electron microscopy facility for physicochemical characterization of nanoparticles in food
CALL CLOSED
METROFOOD-RI 1st open call is promoted as a part of the METROFOOD-PP project (EU H2020 INFRADEV-02-2019 - GA 871083)
With the 1st Open Call METROFOOD-RI subsidises access to its research facilities, allowing users to execute part of their research project. Projects that are well aligned with the aims of METROFOOD-RI and meet technical, scientific and ethical requirements will receive subsidised services (free-of-charge access to the facilities*). The service is addressed to researchers engaged in nanoparticle analysis in food. Remote access to the TEM facility of Sciensano (link), or training and analytical assistance for using the TEM on site will be provided.
About the service and apply here
Deadline for application: 30 Sept. 2020 h. 17:00 CEST (Brussels time)
* due to the COVID-19 sanitary emergency, travel to the facilities might be subject to restrictions.
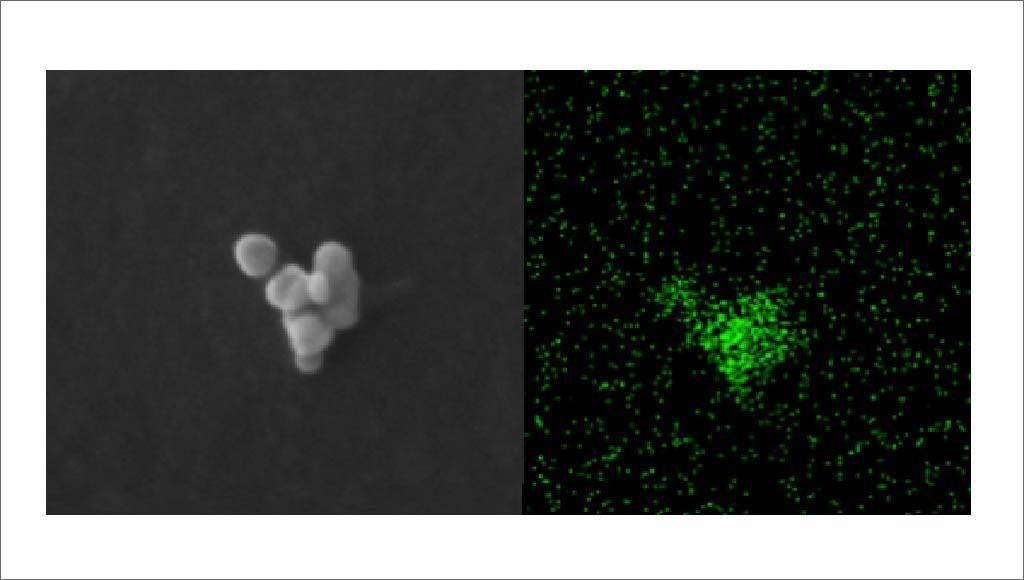
Remote access to transmission electron microscopy (TEM) facility for physicochemical characterization of nanoparticles in food
The service will make a high-end analytical transmission electron microscopy (TEM) available for direct use by external users who request analyses in the framework of the physicochemical characterization of nanomaterials in food. TEM equipment will be available at three levels:
- Analyses performed by Sciensano operators: service fully provided by Sciensano operators, from sample preparation over analysis to report.
- Training & analytical assistance by Sciensano operators: Sciensano provides training in analytical techniques to external (less-experienced) candidates, and experienced or trained candidates can use our advanced equipment on-site with assistance of our operators.
- Remote operation: a remote connection that allows operating the microscope from within another institution will be used to set up an infrastructure where external clients can have remote access to the TEM during a defined amount of time to monitor their own analyses.
Target users: Researchers engaged in nanoparticle analysis in food.
Where? Remote access to the facilities of, or on site assistance at Sciensano - Service Trace Elements and Nanomaterials. Address: Groeselenberg 99, 1180 Ukkel, Belgium.
When? To be defined between the local organisers and the selected applicants
* due to the COVID-19 sanitary emergency, travel to the facilities might be subject to restrictions.
Background - Consumers are exposed to food containing food additives on a daily basis. Several food additives are known to contain nano-sized particles (1-100 nm) with physicochemical characteristics that determine beneficial properties and/or health risks. To balance benefits and risks, regulations are being put in place [EFSA Journal, 2018. 16, EU Reg. 1169/2011, EU Recc. 2011/696, EU Reg. 2015/2283]. All food additives present on the European market must comply with EC Reg. 1333/2008, and are evaluated based on a case-by-case risk assessment performed by the European Food Safety Authority (EFSA). In addition, food additives permitted before 20 January 2009 must go through a new risk assessment by EFSA. EC Reg. 257/2010 set up a program for the re-evaluation of approved food additives in accordance with EC Reg. 1333/2008. Application areas within EFSA’s remit are e.g. novel foods, food contact materials, food/feed additives, and pesticides. For assessing the safety of nanomaterials in these application areas, EFSA issued the guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain [EFSA Journal, 2018. 16(7)]. This Guidance states that all dossiers related to nanomaterials have to be accompanied by detailed information on the particle size distribution and on other parameters of the material obtained through validated methods based on suitable analytical techniques.
The physicochemical characterization of a nanomaterial is needed to decide whether the material has to be considered for nanospecific risk assessment under this Guidance, to determine the physical and chemical identity of the pristine material, to characterize the material in test media used in toxicokinetic and toxicological studies, and to characterize the material in complex matrices e.g. product formulations, needed for exposure assessment [EFSA Journal, 2018. 16(7)]. Several authorities recognize the need for electron microscopic analysis in the control of nanomaterials in the food chain (EFSA, CEN, SCENIHR). Both CEN/TC 352: “Nanotechnologies — Guidance on detection and identification of nano-objects in complex matrices” and “The guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain” [EFSA Journal, 2018. 16(7)] by EFSA argue that all dossiers related to nanomaterials have to be accompanied by detailed information on the particle size distribution and other parameters of the material obtained through validated methods based on suitable analytical techniques, and recommend that particle size distribution should be determined by more than one independent technique, one of which being electron microscopy.
Fee: free of charge access to the facility (with no contribution to eventual travel and subsistence costs)
Eligibility Criteria - The selection procedure will take into account the compliance with the technical, scientific and ethical requirements. Requested analyses have to fit into a scientific project related to food safety. It is recommended that the candidates have previous experience in nano-related analytical techniques. The request has to include clearly defined objectives and time-frame. If the application is positively evaluated, Sciensano operators will schedule a technical-scientific meeting with requester to define the best approach.
Applicants need to register via the dedicated form – click here